A pilot project, also dubbed a pilot experiment or preliminary study, acts like a trial run before you dive into a full-blown research project. It's a crucial step for smoothing out kinks and making sure your research methods are on point, seriously upping your chances of nailing the main study. This initial phase is all about ironing out the feasibility, fine-tuning recruitment tactics, figuring out the right number of participants, and getting the treatment-testing strategy just right. By giving these elements a once-over, a pilot project sets you up for more effective and efficient Phase I clinical trials and other big research gigs.
Introduction to pilot projects
Think of a pilot project as a test drive for your research—it's small-scale and gives you a sneak peek into what's involved in the full deal, like the time, costs, and potential hiccups. These mini versions, packed with elements of quantitative research, let you test-drive everything from the study design to the nitty-gritty of clinical trials. They're crucial for tweaking the big-picture plans and bumping up the odds of your project's success.
Definition of a pilot project
A pilot project is your research's test run, helping you see if your ideas are feasible before going all in. It's where you test out stuff like how to find people for your study, whether your sample size cuts it, and how you might handle the treatments. These projects are big players in Phase I clinical trials, where they help you make all the big decisions about the study's setup and how you'll analyze the data, making sure you're setting yourself up for less risk and solid research.
Benefits of using Bonsai for pilot project
Launching a pilot project can be challenging. Bonsai offers a suite of features to ensure a smooth takeoff of your project.
With Bonsai, teams can set clear objectives, allocate resources, and establish structures to oversee the pilot project's progress. The platform's ability to track tasks, deadlines, and budgets in real-time allows for meticulous monitoring and quick adjustments when necessary. Communication is streamlined within Bonsai, ensuring that all stakeholders are kept informed and can collaborate on refining the project as it evolves.
Bonsai helps your pilot project soar by promoting streamlined communication, adaptable workflows, and data-driven insights. Increase your pilot's success rate and pave the way for a smooth implementation. Bonsai equips teams with the tools to navigate the complexities of pilot projects, from planning to execution, ensuring a solid foundation for future scaling.
Importance of pilot projects in business
In the business world, pilot projects are key. They're like a rehearsal for the major leagues, allowing companies to test the waters of larger projects, sort out recruitment, and nail down how many people they need. These trials are perfect for trying out different scenarios and setting up designs down the road. By running a pilot, businesses can fine-tune their research methods, sharpen their data game, and lay the groundwork for minimizing risks before jumping into big projects or clinical trials.

Key elements of a successful pilot project
A pilot project’s gotta be tight, like a dress rehearsal for the big show. It’s all about setting up a mini-version of your grand plan, making sure you’ve got the right folks on board, and testing the waters with your treatments. Think of it as a sneak peek—like a Phase I trial—to see if your idea’s got a game before you go to the big league. The jackpot? A pilot that can call the shots on how the full-scale project will roll, catching any curveballs early on.
Clear objectives and goals
Kick things off with a pilot that’s like a mini-mission for your mega research dreams. It’s a small gig that lays the bricks for the big builds, using all the number-crunching tricks to test your moves. You’ll play matchmaker, pairing up the right number of peeps for your Phase I jam. Basically, these test runs are your blueprint for the monster studies ahead.
Defined scope and parameters
This part focuses on kicking off a pilot experiment as a stepping stone to a larger research project. It’s about getting a clear picture from the get-go, from figuring out how to bring people in, to sizing up the crowd, to making sure the study design is practical. It also covers the nitty-gritty of Phase I clinical trials, like how you’ll test treatments and handle the stats.
Stakeholder engagement
Getting stakeholders involved is key in these early stages. Their input is invaluable for shaping the pilot study and figuring out the scale of the project. Their feedback is especially important in Phase I trials, where their insights can significantly impact how treatments are tested and how the overall study is designed.
Resource allocation
Before diving into a full-scale project, it’s smart to run a pilot experiment or small-scale study. This helps you refine your study design and understand the resources you’ll need, like how many people to include and what challenges you might face. These preparatory steps are vital, especially in clinical trial design, where a well-planned Phase I trial can save money and optimize resources. Plus, these early trials are crucial for effective treatment testing and accurate data analysis.
Planning a pilot project
Planning a pilot project is all about nailing the details. You start with a pilot experiment as a smaller, test-run study to see if the full-blown project will fly. This step is key for checking if your study design, sampling methods, and recruitment strategies are up to scratch. In this stage, you're zoning in on stuff like how big your sample size needs to be, how you'll test treatments, and other big-deal guidelines for Phase I clinical trials.
Plus, you've got to plan out your statistical analysis with an eye on the quirks of quantitative research. These early tests are gold for tweaking your plans for the bigger trials, cutting down risks, and polishing up the quality of your final project.
Identifying the project needs
At the very start of your full-scale research, a pilot experiment is a must to check if your research plans hold water. This small-scale preliminary study helps you shape up the study design, figure out if your sample size is enough, and guess how many folks will be keen to join in based on how they've reacted before.
This early run also steers your strategy for Phase I clinical trials. You'll be looking at how to refine your treatment tests, setting up a smart system for gathering data, and planning your stats analysis. Solid planning here is what makes or breaks your bigger project later on.
Choosing the right team
Pulling off a successful pilot experiment or small-scale study means picking the perfect team. You need pros who are sharp at crafting the study design and who can handle deep-dive statistical analysis. It’s crucial to have folks who are good at getting people on board because the number of participants can make or break your full-scale project.
Plus, you need someone who can manage a Phase I clinical trial and maybe even lead more pilot trials down the line. The right crew will be all over the treatment testing and ensure everything flows smoothly from this preliminary study to the full-scale research gig.
Setting a realistic timeline
Getting a research study off the ground starts with a feasibility study to see if your ideas work in the real world. After that, you usually jump into a pilot experiment. The timeline for this initial phase can vary a lot depending on what you're researching, how you're testing treatments, how fast you can get people involved, and how many people you need.
Once you nail the pilot trials, you move on to a full-scale project or a Phase I clinical trial, which involves more in-depth quantitative research and statistical analysis. The length of this next phase can stretch from a few months to years, influenced by how complex your research is and what the initial tests tell you.
Executing a pilot project
Before undertaking a full-scale research project, it is necessary to conduct a pilot experiment. This small-scale study serves as a preliminary study, testing the feasibility of the trial design, recruitment strategies, and statistical analysis methods. Crucially, it can help to determine an appropriate sample size for quantitative research.
In medical scenarios, this early-stage investigation often resembles a Phase I clinical trial. Pilot trials play a foundational role in refining treatment testing protocols. Ultimately, the insights drawn from well-executed feasibility studies in these stages play a critical role in the success of larger projects.
Implementing the Plan
For a successful full-scale research project, a small-scale study or feasibility study would be the next step. This involves a pilot experiment that aids in the refinement of the study design and reassesses sample size requirements.
Along with this, the feasibility studies would help optimize the recruitment strategy and treatment testing procedures. These pilot trials can also be considered as the foundation stone of the potential Phase I clinical trial.
Finally, a preliminary quantitative research will be carried out to confirm the expectations on which the statistical analysis of the full-scale research project will be eventually based.
Monitoring and controlling the project
The monitoring and controlling aspects of the project will involve a systematic pilot experiment, intended as a small-scale study and preliminary to any full-scale research project implementation. This approach allows us to ensure the project's feasibility and address potential issues prior to any extensive involvement.
Key components of this portion of the project will include establishing an optimal sample size for recruitment, strategy for treatment testing, and creating an adequate design for both study and clinical trial. A phase I clinical trial will also take place as part of feasibility studies and pilot trials.
Furthermore, careful quantitative research and statistical analysis will aid in data interpretation, providing valid and reliable results, thereby ensuring project success.
Role of technology in pilot projects
Technology is a game-changer in pilot projects, from designing the study to crunching numbers and testing treatments.
During a preliminary study, tech tools help manage and analyze data, giving you a full picture of your concept. This deep dive into data can shape your recruitment strategies too.
In Phase I clinical trials and other early-stage studies, technology figures out the sample size you need to get results that apply to a bigger group. It also makes it easier to scale up to a full-scale project, handling more data and tougher analyses with ease.
Use of project management tools: Bonsai, Trello, Asana, and Jira
Project management tools like Bonsai, Trello, Asana, and Jira are lifesavers for planning and running research projects. They’re especially handy for preliminary studies or feasibility studies, where keeping track of tasks, hitting deadlines, and measuring progress is crucial.
.webp)
These tools help you plan systematically, making study design and recruitment processes smoother. They’re also great for bigger projects and Phase I clinical trials, offering a space for team communication, file sharing, and task updates. Using these tools in smaller research efforts like pilot studies ensures tasks are assigned and objectives are met on time.
Communication tools: Bonsai, Slack and Microsoft Teams
Bonsai, Slack and Microsoft Teams are must-haves for team communication in any research setup, whether it’s a preliminary study, small-scale study, or full-scale project. They streamline coordination in key areas like recruitment, treatment testing, and study design improvement. For example, in a Phase I clinical trial, these tools help with discussions about sample size and statistical analysis. With Slack and Teams, running pilot trials or feasibility studies becomes more efficient, letting researchers zero in on the important parts of their work.

The Bonsai platform offers a centralized space where team members can engage in real-time conversations, share updates, and provide feedback directly within the context of their projects. With features like integrated messaging, teams can quickly resolve queries, discuss task details, and make decisions without leaving the platform.
Evaluating the success of a pilot project
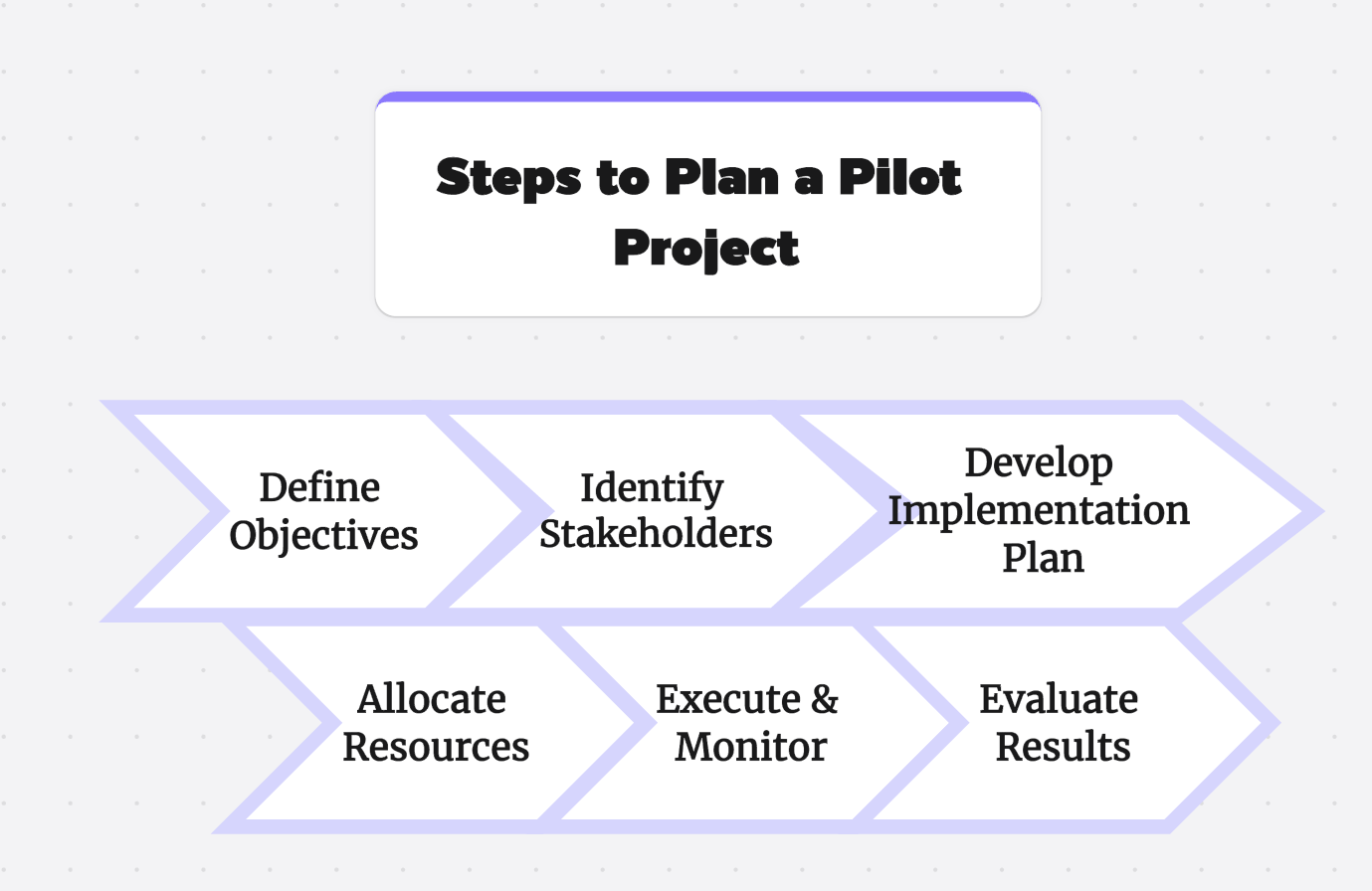
Evaluating the success of a pilot project relies on several key stages. The first stage is the design of the pilot experiment, including careful planning of recruitment and determination of the sample size. This small-scale study serves as a preliminary study to guide the feasibility of a full-scale research project.
In a Phase 1 clinical trial, for instance, the main focus is on treatment testing and conducting quantitative research. The data collected is then subjected to robust statistical analysis. Hence, the success of these pilot trials and feasibility studies primarily hinges on the rigor of the study design and the accuracy of the results analyzed.
Measuring outcomes against objectives
Pilot trials and feasibility studies have paved the way for larger, full-scale research projects. The outcomes of these preliminary tests are often measured against established objectives to monitor progress and identify areas for improvement.
A critical part of study design, particularly in clinical trial design, is the successful recruitment and managing a sufficient sample size. This can majorly influence the reliability and accuracy of results.
Treatment testing is another critical objective measured against. It incorporates statistical analysis of data collected during a study, like a Phase I clinical trial, to evaluate whether objectives are being achieved.
Feedback collection and analysis
In the preliminary stages of a research project, often a pilot experiment or small-scale study is conducted. This serves as a feasibility study to examine the practical implications of the full-scale research project. It also helps determine key elements such as the study design, recruitment strategy, and sample size.
Typically in a Pilot trial, Phase I clinical trial or in treatment testing, one key component is collection and analysis of feedback. This feedback can further inform the feasibility studies and guide any necessary changes to the study design or statistical analysis methods before scaling up to a larger quantitative research.
Therefore, carefully collecting and analyzing feedback is of paramount importance to ensure the success and validity of the eventual full-scale project.
Common hurdles in pilot projects & avoiding them

Pilot studies, you know, those trial runs? They hit snags like finding enough folks to join, or nailing a clinical trial design that actually works. But hey, roll up your sleeves, plan ahead, and you can tackle these head-on. Like, get your recruitment pitch perfect, and you’ll have people lining up. Make your study design tight, and you’ll get the good stuff—data that means something. And a pilot trial? That’s your crystal ball, showing you if you’re ready for the big leagues.
Scope Creep: Keeping it in check
Ever seen a project balloon into a beast you can’t tame? That’s scope creep. It’s when your study starts growing wild, sprouting bits that were never part of the plan. To keep your research from turning into a wild goose chase, start with a pilot. It’s like a sandbox where you can play with your ideas—figure out the best way to get people in, how many you need, and how to test your treatments. These test runs are gold for keeping your study on track and making sure you’re not biting off more than you can chew.
Aligning everyone on board
When everyone’s rowing in different directions, your pilot studies going nowhere fast. Stakeholder misalignment is like having cooks spoil the broth with their own recipes. You’ve got to get everyone on the same page, especially when you’re deciding how big your study should be or how you’re gonna test things out. It’s all about chatting it up and working together, so when you dive into Phase I trials, you’re all swimming in sync.
Stretching resources thin
Starting out, our pilot study was like trying to make a feast with a shoestring budget. We were juggling trying to get enough people and not blowing through our funds. The small-scale study we did was a juggling act—making sure our stats were solid without running dry. Looking ahead, if we want our full-scale research or Phase I trials to really rock, we need the dough to back it up. We’re talking big sample sizes, thorough testing, and deep dives into the data.
Conclusion: The value of pilot projects for agencies
Pilot projects? They’re the secret sauce for agencies. They let you dip your toes in the water with new ideas before you go all in. It’s like a test run to see if your plan’s got legs, making sure you’re not barking up the wrong tree with how you’re getting people on board or figuring out how many folks you need. Think of feasibility studies as your backstage pass to the big leagues of Phase I clinical trials. They’re your chance to make sure your stats game is strong and your data makes sense. Bottom line: pilot projects are your best buds for nailing that solid research and setting the stage for the big show.







